We are all familiar with the three primary nutrients required by plants: nitrogen (N), phosphorus (P), and potassium (K). This is because the concentrations are printed on the labels of most fertilizer bags found in farm and garden stores.
When your soil is deficient in all three elements, the relative contribution of each element becomes less important than if your soil is deficient in only one or two of the three elements. When you order a truckload of fertilizer from your local supplier, you may have more control on the balance of N, P, and K to purchase since different fertilizer formulations can be mixed or blended.
If you buy a 50-pound bag of fertilizer, the N, P, and K values let you know how much of that total weight is divided into N, phosphorus as P2O5, and potassium as K2O. A common fertilizer blend is 10-10-10, meaning that 10% of the material contains N, 10% contains P2O5, and 10%, contains K2O, which would be 5 pounds of each.
Mining for P
Since P can be derived from different sources, the standard fertilizer form of P2O5 has been internationally accepted. Phosphorus is typically sourced as triple super phosphate with N-P-K of 0-45-0; monoammonium phosphate with N-P-K of 11-48-0; diammonium phosphate with N-P-K of 18-46-0; or rock phosphate with 14% to 35% P and relatively low immediate plant availability.
All P fertilizer sources are mined from geologic deposits of mostly apatite, which is a calcium phosphate mineral extracted from sedimentary marine deposits and some igneous sources. Acidificaiton and processing of these minerals produces a readily available P fertilizer source; however, there is a limit to the amount of P mining that can occur globally.
Phosphorus is also recycled from plant residues and animal excreta to soil. Consider that 7 to 13 pounds of P is removed from every 1,000 pounds of beef cattle harvested. A key issue with P cycling is the strong chemical binding with soil minerals that sequester P away from plants.
Excess application
Phosphorus is vital to plants so that they can photosynthesize, metabolize sugars, store and transfer energy, divide and enlarge cells, and transfer genetic information. When soil is low in available P, fertilization promotes root growth and winter hardiness, stimulates tillering, and hastens maturity.
Applying P fertilizer indiscriminately without regard to the amount of P readily available in soil is costly; it is not a wise use of limited resources and can be detrimental to the environment. Phosphorus runoff from overloaded soils has caused harmful algal blooms in surface waters. Loading surface waters with N and P leads to rapid and excessive growth of algae that die and decompose. This decomposition consumes oxygen in the water, leading to anaerobic conditions. Of particular concern are blooms of cyanobacteria, also known as blue-green algae, which thrive in waters with high P levels. These blooms can release toxins into the water, negatively impacting human and animal health and disrupting aquatic ecosystems.
Soil testing for P is the best way to know if fertilizers should be applied to enhance production on your farm. Depending on which state you reside in and which analytical lab you use, the extraction of available P can vary. Some extractants will be more appropriate in your particular soil than others.
Sufficient P levels have typically been determined in each state or within a region from a series of plant-growth response trials. These trials expose a crop of interest such as corn, wheat, or forage to a gradient of P fertilizer levels and yields determined to establish the optimum rate of P fertilization. Following several dozen trials with varying levels of available soil P — which was based on soil testing prior to the growing season — critical soil-test P levels were identified to divide yield-responsive soils from unresponsive soils. In North Carolina, sufficiency level of P from Mehlich-3 extraction is approximately 60 ppm for many crops.
It varies with depth
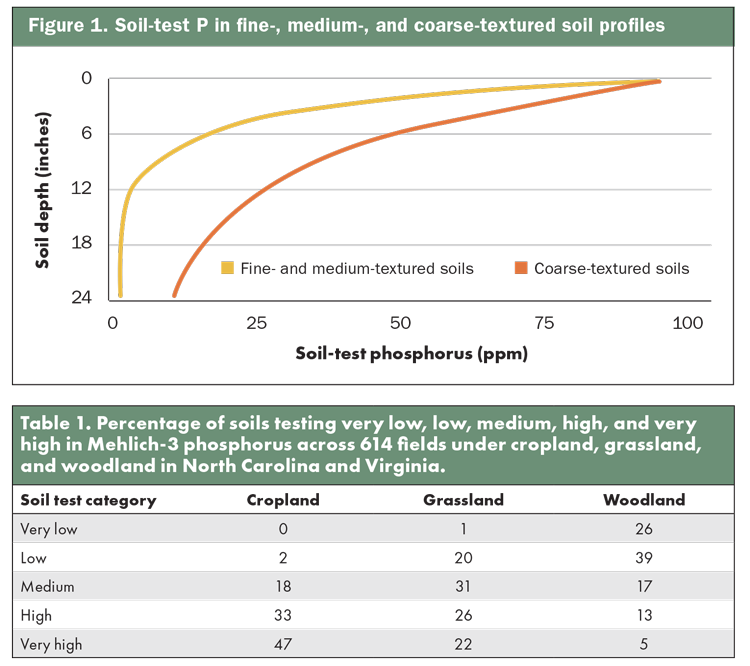
In a survey of 56 farms across North Carolina and Virginia, my research team explored the distribution of soil-test P from different soil depths and land uses, resulting in the testing of 614 soil profiles (see table). The figure indicates the median P levels when soils were sorted by fine, medium, and course textures. In general, soil-test P was greatest from 0 to 4 inches and declined dramatically at depths of 4 to 12 inches and 12 to 24 inches. The vast majority of roots able to extract P will be concentrated near the surface in the top foot of soil, so the correspondence between where nutrients are available and where roots proliferate is fortunate.
Clearly, sandier soils tended to have greater P concentrations at all depths. This is because P binds with clay minerals and is not readily available, so soil-test P was lower in fine- and medium-textured soils. On the other hand, sandy soils don’t have as much affinity for binding P to minerals. Clayey subsoil is a key mechanism for binding P and not allowing it to leach below the rooting zone.
When soil-test P data from the 0-to-4-inch depth were sorted by land use, only 20% of soils under cropland were deficient in P, while 52% of soils under grassland were deficient. Under woodland, 82% of soils were deficient. These data suggest that a large fraction of soils under cropland and grassland could be considered well-supplied with P and there is little need for immediate and high fertilizer inputs. However, soil testing is the surest approach to know the level of available soil P on your own farm.
In summary, P is a vital nutrient required in relatively large quantities. Available P in soil is only a fraction of total P present, and this fraction depends on soil texture. Optimizing forage production requires P fertilization when soil-test values are very low, low, or medium. However, there is no economic benefit from P fertilization when soil-test values are high or very high. Environmental health can be compromised when routine fertilization occurs without soil testing to guide application rate and frequency.
This article appeared in the April/May 2025 issue of Hay & Forage Grower on page 10-11.
Not a subscriber?Click to get the print magazine.

